 |
Knoxville TN (SPX) Jun 18, 2010 As carbon dioxide continues to burgeon in the atmosphere causing the Earth's climate to warm, scientists are trying to find ways to remove the excess gas from the atmosphere and store it where it can cause no trouble. Sigurdur Gislason of the University of Iceland has been studying the possibility of sequestration of carbon dioxide (CO2) in basalt and presented his findings to several thousand geochemists from around the world at the Goldschmidt Conference hosted by the University of Tennessee, Knoxville, and Oak Ridge National Laboratory. Carbon sequestration is currently the most promising way to reduce greenhouse gases. Gislason leads an international team of scientists on the Carbfix Project, which aims at pumping carbon deep underground in southwest Iceland where it will mix with minerals and become rock. The project's goal is to find a storage solution that is long lasting, thermodynamically stable and environmentally benign. An Icelandic geothermal plant is now hosting the pilot program. Gislason's project involves capturing and separating flue gases at the Hellisheidi Geothermal Power Plant, transporting the gas, dissolving it in water, and injecting it at high pressures to a depth between 400 and 800 meters into a thick layer of basalt. Then he and his coworkers will verify and monitor the storage. Carbon dioxide mixed with water forms carbonic acid (also known as carbonated water or soda water), which percolates through the rocks, dissolving some minerals and forming solid carbonates with them, thereby storing the carbon dioxide in rock form, said Gislason. If successful, Gislason said, the experiment will be scaled up and can be used wherever carbon dioxide is emitted. Currently, carbon may be captured as a byproduct in processes such as petroleum refining. It can be stored in reservoirs, ocean water and mature oilfields. However, many experts fear that CO2 may leak over time. Storage of CO2 as solid magnesium carbonates or calcium carbonates deep underground in basaltic rocks may provide a long-term and thermodynamically stable solution.
Share This Article With Planet Earth
Related Links University of Tennessee at Knoxville Carbon Worlds - where graphite, diamond, amorphous, fullerenes meet
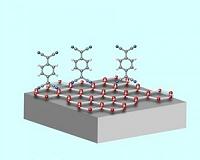 Doping Graphene
Doping GrapheneCollege Park MD (SPX) Jun 07, 2010 An organic molecule that has been found to be effective in making silicon-based electronics may be viable for building electronics on sheets of carbon only a single molecule thick. Researchers at the Max Plank Institute for Metals Research in Stuttgart report the advance in a paper appearing online in the journal Physical Review B on June 1. Ultrathin carbon layers known as graphene show p ... read more |
|
| The content herein, unless otherwise known to be public domain, are Copyright 1995-2010 - SpaceDaily. AFP and UPI Wire Stories are copyright Agence France-Presse and United Press International. ESA Portal Reports are copyright European Space Agency. All NASA sourced material is public domain. Additional copyrights may apply in whole or part to other bona fide parties. Advertising does not imply endorsement,agreement or approval of any opinions, statements or information provided by SpaceDaily on any Web page published or hosted by SpaceDaily. Privacy Statement |