 |
Washington (AFP) Oct 27, 2008 Starting anti-retroviral treatment earlier sharply improves survival rates for people infected with HIV, according to a new study. Researchers say analysis of thousands of HIV-positive patients between 1996 and 2006 found a 71-percent higher risk of death for those who delayed treatment compared with those initiating early highly active anti-retroviral therapy (HAART), the National Institutes of Health (NIH) reported Sunday. The study, led by Mari Kitahata of the University of Washington-Seattle, looked at 8,374 patients whose CD4+ T-cells -- key "helper" cells in the body's immune system -- were between 351 and 500 cells per square millimeter in blood. Thirty percent of subjects in the study began taking HAART right away, while 70 percent waited until their T-cell count dropped below 350, when the immune system is considered seriously compromised. "The researchers found a 71 percent higher risk of death for patients who deferred treatment rather than initiating HAART, suggesting that therapy should begin at an earlier stage of HIV disease than currently recommended," NIH said in a statement. The optimal timing for the introduction of HAART therapy -- a combination of at least three HIV medicines -- has been a point of contention for more than a decade, with some doctors suggesting holding off treatment as long as possible in order to spare patients the side effects of AIDS drugs. Up to now, guidelines had recommended that HIV treatment begin when a person's T-cell count dropped below 350. "The data are rather compelling that the risk of death appears to be higher if you wait than if you treat," Anthony Fauci, director of NIH's National Institute of Allergy and Infectious Diseases which sponsored part of the research. The findings were presented Sunday at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) in Washington. Lead researcher Kitahata said the findings were striking because of the "magnitude" of the difference in survival rate between the two groups, according to USA Today. "Seventy percent is a significant and substantial increase in the risk of death," the daily quoted Kitahata as saying. According to the World Health Organization, 33 million people around the world are infected with the AIDS virus, mostly in sub-Saharan Africa. One million HIV-infected people live in the United States. Some two million people died worldwide of AIDS in 2007. On Monday the world body sharply cut an earlier mortality forecast, saying the latest forecast now expects deaths to rise from 2.2 million in 2008 to a maximum of 2.4 million in 2012, before declining to 1.2 million in 2030. An earlier WHO projection had HIV and AIDS deaths would rise to 6.5 million in 2030. Related Links Epidemics on Earth - Bird Flu, HIV/AIDS, Ebola
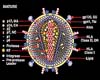 Geneva (AFP) Oct 27, 2008
Geneva (AFP) Oct 27, 2008The number of deaths arising from HIV and AIDS is expected to peak in the next five years, the World Health Organization said Monday, as it sharply cut an earlier mortality forecast. |
|
| The content herein, unless otherwise known to be public domain, are Copyright Space.TV Corporation. AFP and UPI Wire Stories are copyright Agence France-Presse and United Press International. ESA Portal Reports are copyright European Space Agency. All NASA sourced material is public domain. Additional copyrights may apply in whole or part to other bona fide parties. Advertising does not imply endorsement, agreement or approval of any opinions, statements or information provided by Space.TV Corp on any Web page published or hosted by Space.TV Corp. Privacy Statement |